Long live the lithium-ion battery
News category: Newnano
 Imagine this scenario: it’s midnight, and you’re chasing a deadline. Your fingers are flying over the keyboard, and as you pause to take a sip of coffee, your laptop sweetly informs you that it’s shutting down because your battery is flat. For most of us, that’s not hard to imagine. Laptops and cell phones that run out of battery power are, inconveniently, part of our lives. Most portable electronic devices, as well as electric cars, are powered by rechargeable lithium-ion batteries. Because of their critical function, nanotechnologists are focusing their gaze on finding effective improvements to enhance the performance and lifespan of these batteries.
Imagine this scenario: it’s midnight, and you’re chasing a deadline. Your fingers are flying over the keyboard, and as you pause to take a sip of coffee, your laptop sweetly informs you that it’s shutting down because your battery is flat. For most of us, that’s not hard to imagine. Laptops and cell phones that run out of battery power are, inconveniently, part of our lives. Most portable electronic devices, as well as electric cars, are powered by rechargeable lithium-ion batteries. Because of their critical function, nanotechnologists are focusing their gaze on finding effective improvements to enhance the performance and lifespan of these batteries.
Lithium-ion batteries work on the principle of lithium ions (a charged form of lithium atoms) that move back and forth from the negatively charged end (the anode) and the positively charged end (the cathode) during discharge and recharging of the battery. Both the cathode and anode can be made of various materials, and manipulation of the structure and chemical properties of these materials can greatly affect the number of recharge cycles and lifetime of a battery.
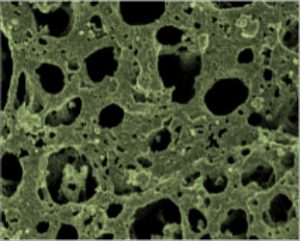
Based at the Council for Scientific and Industrial Research (CSIR) and the University of Pretoria’s department of chemistry, one of the nanotechnology research activities of Prof Kenneth Ozoemena’s research group is to improve the performance of the cathode electrode of lithium-ion batteries. Their research has recently culminated in an international patent. They have improved cathodes made of lithium manganese oxide (LiMn2O4), by modifying the structure with nickel nanoparticles (as you can see in the microscope image for nickel-modified LiMn2O4). The nano-structured particles serve to increase the surface area so that more reactions, such as the back-and-forth movement of the lithium ions, can take place. In other work they have shown that the addition of various compounds, such as nickel, iron, and aluminium to the LiMn2O4 also increases the operation efficiency and lifetime of the batteries.
South Africa has the largest manganese deposits in the world, so promoting technology that uses this material could have valuable economic implications for the country. With the help of nanotechnology and our metal reserves we may still experience some valuable breakthroughs in storable energy.
Writer: Febe Wilken
